President Trump hailed the CDC's vote to stop vaccinating all newborns against Hepatitis B while claiming babies are at 'NO RISK' of contracting the virus.
Trump issued a post on Truth Social that also announced a presidential memorandum to fast-track a full review of global vaccine schedules.
'Today, the CDC Vaccine Committee made a very good decision to END their Hepatitis B Vaccine Recommendation for babies, the vast majority of whom are at NO RISK of Hepatitis B, a disease that is mostly transmitted sexually, or through dirty needles,' Trump wrote.
'The American Childhood Vaccine Schedule long required 72 “jabs,” for perfectly healthy babies, far more than any other Country in the World, and far more than is necessary. In fact, it is ridiculous!'
According to John Hopkins University, Hepatitis B can be transmitted through close contact - with minor cuts or even microscopic amounts of blood on a shared surface, for example - to an infant by a caregiver or household member with an infection, even when the infected person is asymptomatic.
Trump then mentioned that 'Many parents and scientists have been questioning the efficacy' of the current US vaccine schedule and ordered a 'FAST TRACK' of an evaluation that would align the US with other countries.
'That is why I have just signed a Presidential Memorandum directing the Department of Health and Human Services to “FAST TRACK” a comprehensive evaluation of Vaccine Schedules from other Countries around the World, and better align the U.S. Vaccine Schedule, so it is finally rooted in the Gold Standard of Science and COMMON SENSE!' he said.



'I am fully confident Secretary Robert F. Kennedy, Jr., and the CDC, will get this done, quickly and correctly, for our Nation’s Children.'
Health and Human Services Secretary Robert F Kennedy Junior's hand-picked panel made the recommendation Friday after delaying the vote yesterday.
Members of the CDC's Advisory Committee on Immunization Practices (ACIP) voted eight to three to recommend that the shot, which protects against a potentially life-threatening liver infection, should no longer be given to all newborns within 24 hours of birth.
Instead, they recommended that, for mothers who did not test positive for hepatitis B, they should consult with their doctor on whether or not to vaccinate their newborn against the disease.
They also voted to recommend that the initial dose of the hepatitis B vaccine should not be administered earlier than two months of age.
The recommendation is not binding, but the committee's guidance typically carries significant influence and is historically followed by doctors, hospitals and major medical organizations.
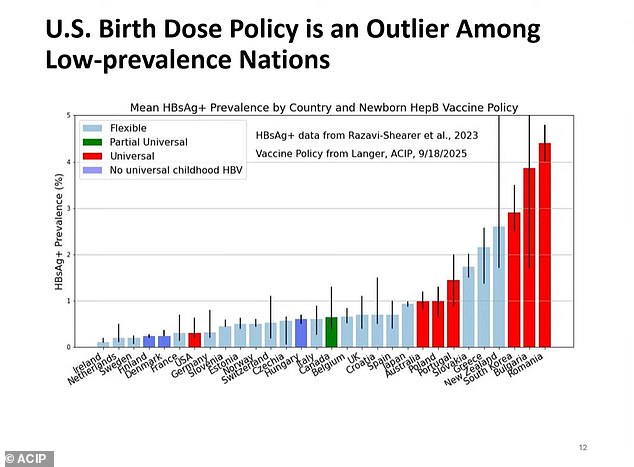
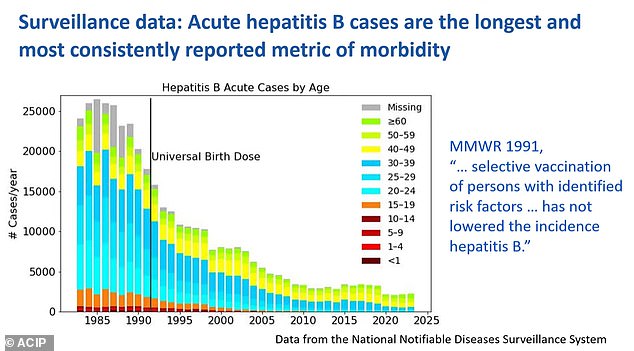
Hepatitis B is a viral infection that attacks the liver, potentially causing lifelong scarring, liver failure and cancer. It is incurable.
The virus is spread via contact with infected blood and body fluids and is able to survive on surfaces for up to a week.
In infants, up to 90 percent of those who are infected develop chronic hepatitis B, which can lead to severe complications and death later in life.
Since 1991, the US has vaccinated against the disease using a three-dose vaccine administered within 24 hours of birth for newborns, regardless of the mother's hepatitis status, and at one month and at six months of age.
Research has shown the vaccine is up to 90 percent effective at preventing mother-to-child transmission if administered within the first 24 hours after birth.
The new recommendation may move back the second and third doses by at least two months.

At today's meeting, among the three members to vote against the new recommendation was Dr H. Cody Meissner, one of the committee's two pediatricians, who warned as he voted that the committee was 'doing harm' by changing its recommendation.
The updated recommendation reads: 'For infants born to [hepatitis B negative] women: ACIP recommends individual-based decision-making in consultation with a healthcare provider, for parents deciding when or if to give the [Hepatitis B vaccine], including the birth dose.'
It added: 'Parents and healthcare providers should consider vaccine benefits, vaccine risks and infection risks.
'For those not receiving the [Hepatitis B vaccine] birth dose, it is suggested that the initial dose is administered no earlier than two months of age.'
The committee still recommends that newborns born to mothers who have tested positive for hepatitis B receive the vaccination at birth.
The committee has also voted to recommend that parents and healthcare providers consider blood tests for children to assess their immunity against the virus after their first dose to confirm whether they need further inoculations.
Read more- Could the CDC's controversial hepatitis B vaccine decision risk a surge in newborn infections?
- Did President Trump just spark a vaccine revolution with his call to delay the Hepatitis B shot for newborns?
- Could a game-changing hepatitis B vaccination policy alter infant health across the US?
- Did a secret CDC study shockingly reveal a 1,135% autism risk increase from newborn hepatitis vaccines?
- What urgent precautions are offered to mothers and babies possibly exposed to hepatitis B at Nepean Hospital?
Post a Comment for "Trump hails CDC vaccine decision while claiming babies are at 'no risk of hepatitis B'"